Welcome to GFP Biotechnology
.
History of GFP
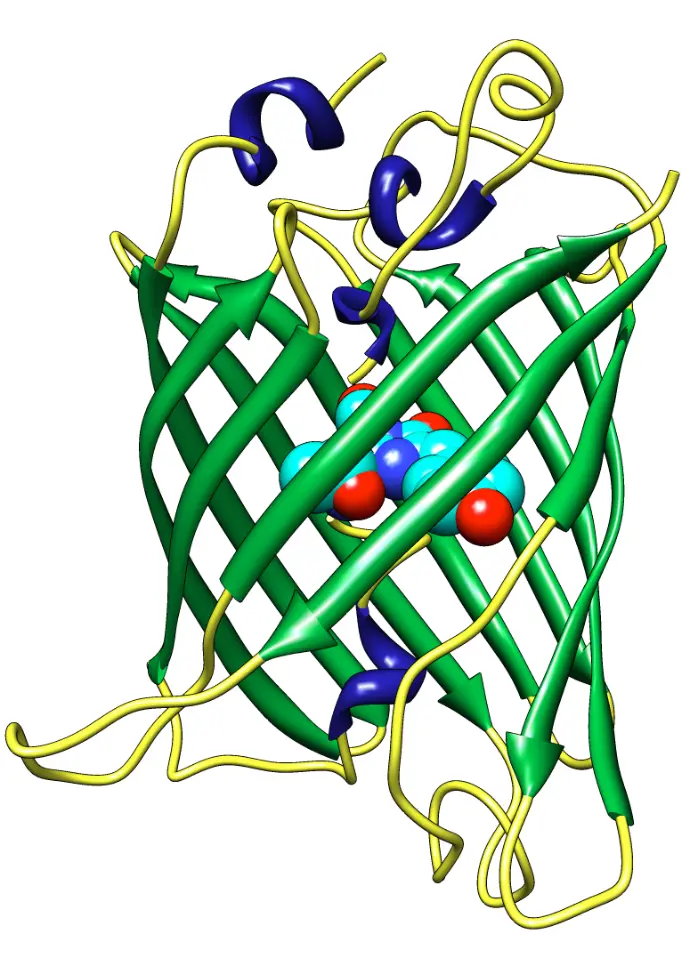
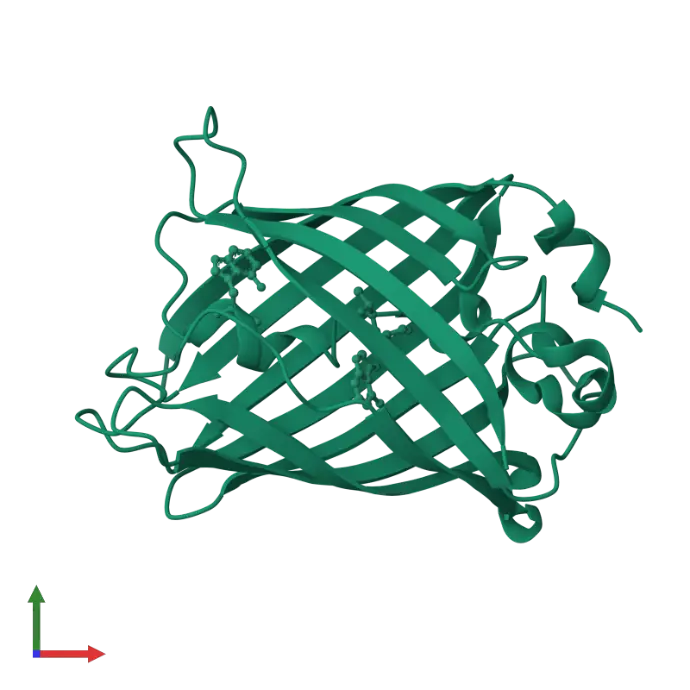
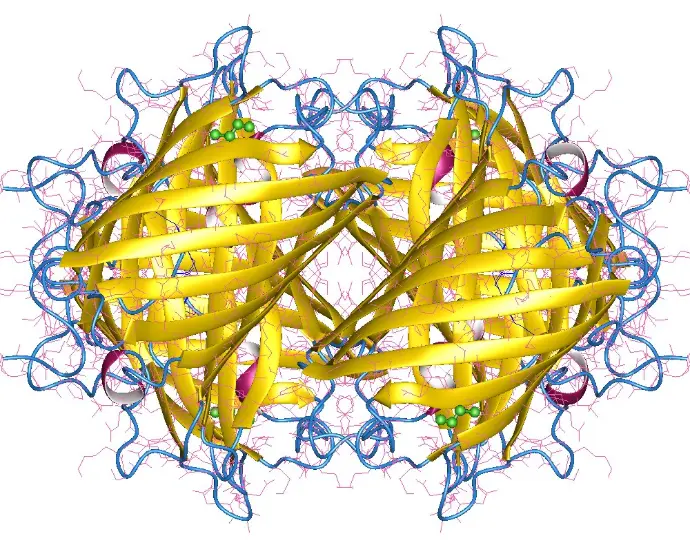
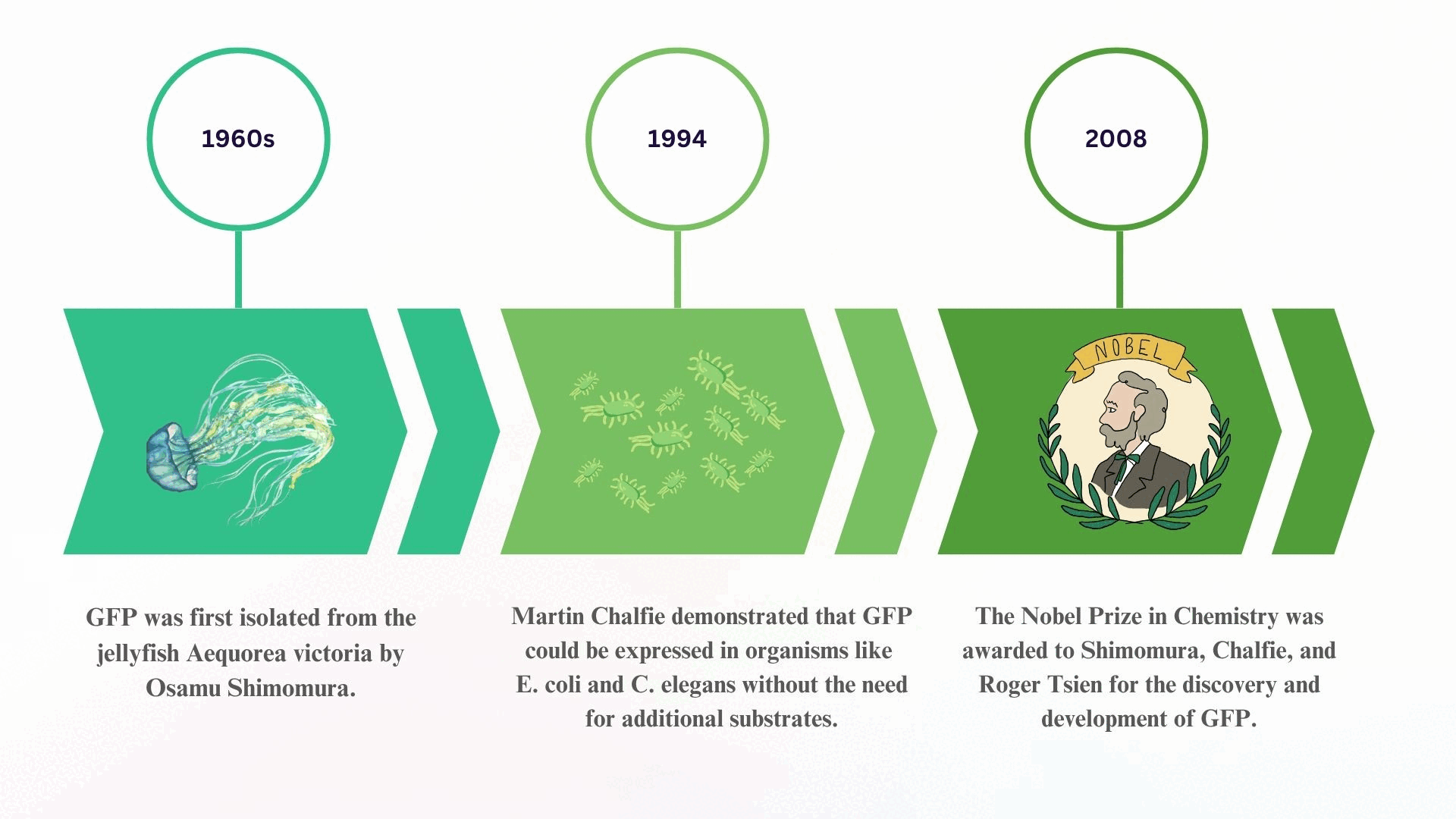
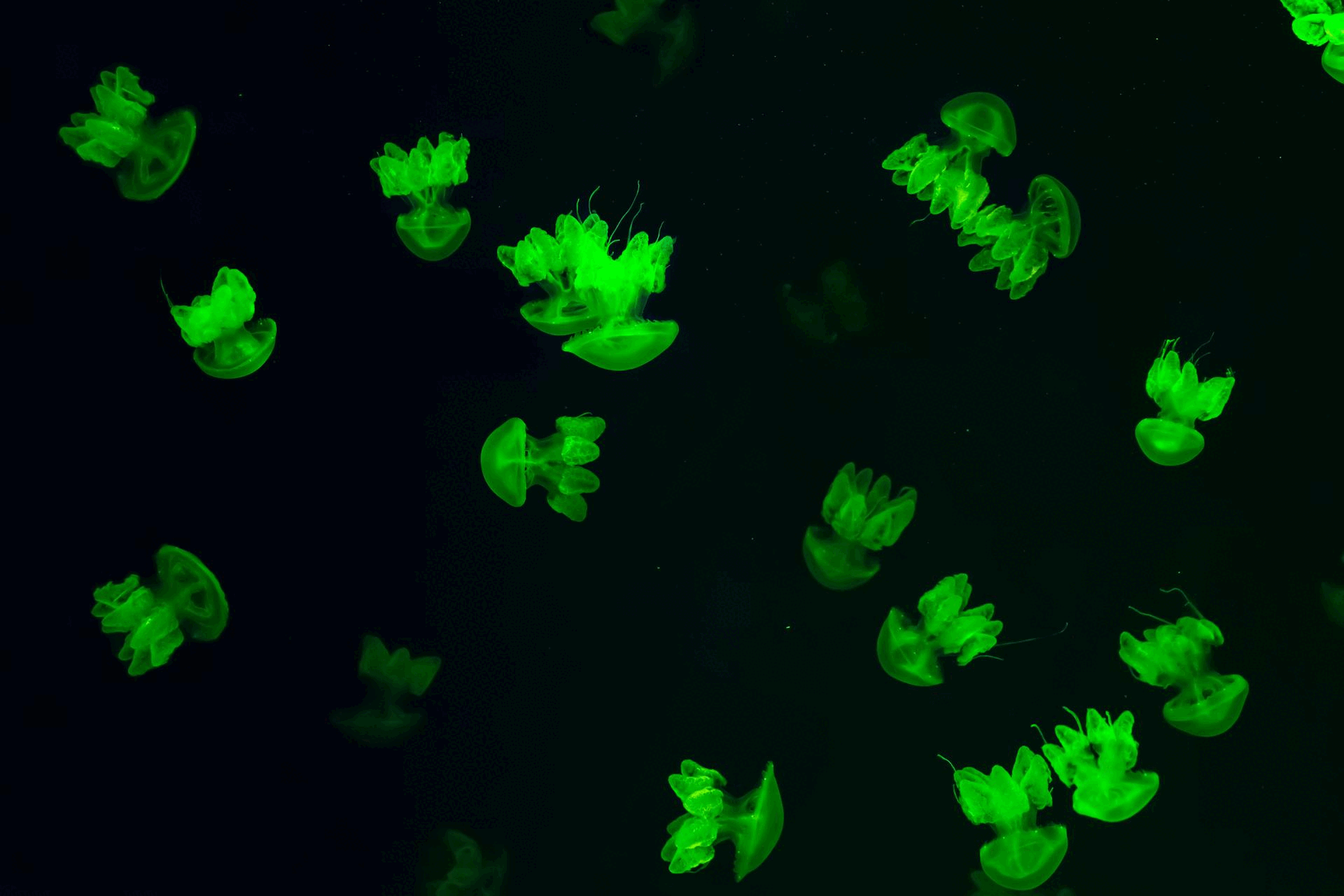
The story of Green Fluorescent Protein (GFP) began in the 1960s, when Osamu Shimomura successfully isolated the glowing protein from the jellyfish Aequorea victoria, unlocking the door to natural bioluminescence.
Decades later, in 1994, Martin Chalfie made a groundbreaking discovery: GFP could be expressed in other living organisms, such as E. coli and C. elegans, without the need for any external cofactors making it a powerful tool for live-cell imaging.
This revolutionary work culminated in 2008, when Shimomura, Chalfie, and Roger Tsien were awarded the Nobel Prize in Chemistry for their collective contributions to the discovery, application, and enhancement of GFP in biological research.
Functions and Applications of Green Fluorescent Protein (GFP)
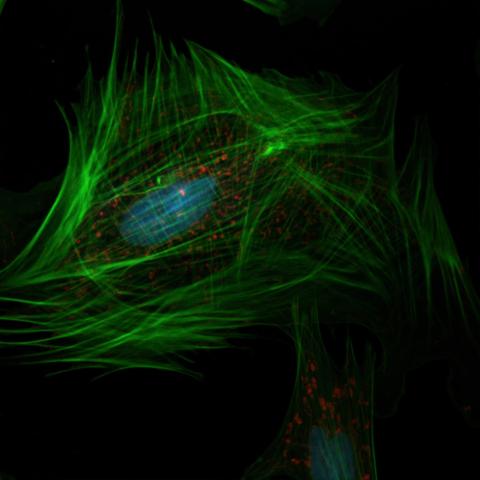
GFP emits green fluorescence (peak emission at ~509 nm) without needing any cofactors.
It can be fused to other proteins to track their expression and localization in living cells.
Used in research fields such as:
Cell biology
Neuroscience
Cancer research
Genetic engineering
Variants of GFP now exist with different colors (e.g., YFP, CFP, RFP), enabling multicolor imaging in cells.Read more
has become an essential tool in modern biological and biomedical research.
Its ability to emit green fluorescence without any additional cofactors makes it ideal for use in live-cell studies. Here are the main applications of GFP in research:
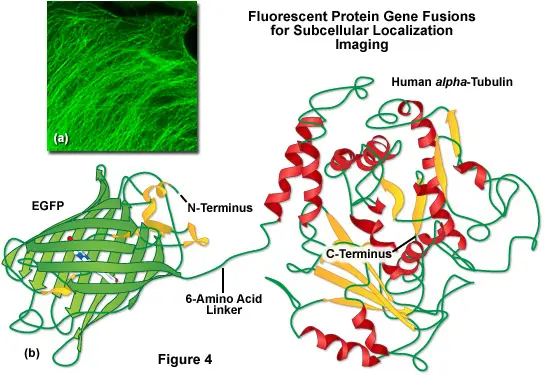
GFP is commonly used as a reporter gene to study gene expression patterns in living cells and organisms. By linking GFP to a gene of interest, researchers can visualize when and where that gene is active by simply observing the emitted fluorescence.
GFP allows for real-time tracking of individual cells or populations of cells over time. This is crucial in fields such as developmental biology, immunology, and cancer research, where understanding cell migration and differentiation is key.
Since the discovery of Green Fluorescent Protein (GFP), scientists have developed numerous variants and engineered mutants to expand its usefulness in research. These modifications have improved the brightness, photostability, and spectral properties of GFP, making it suitable for more advanced and diverse applications.
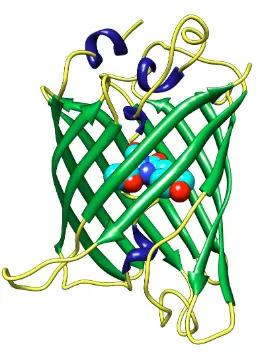
Enhanced GFP (eGFP)
eGFP is a mutant version of wild-type GFP that offers greater brightness and improved folding efficiency at 37°C, making it ideal for use in mammalian cells. It is the most widely used variant in cell biology and molecular imaging.
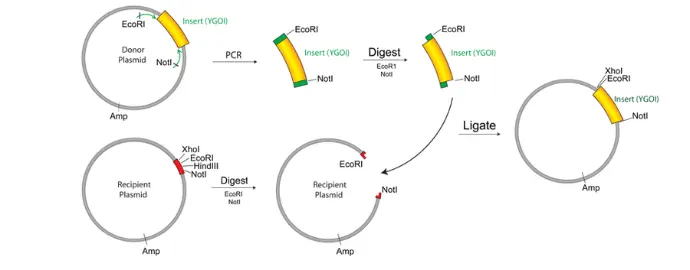
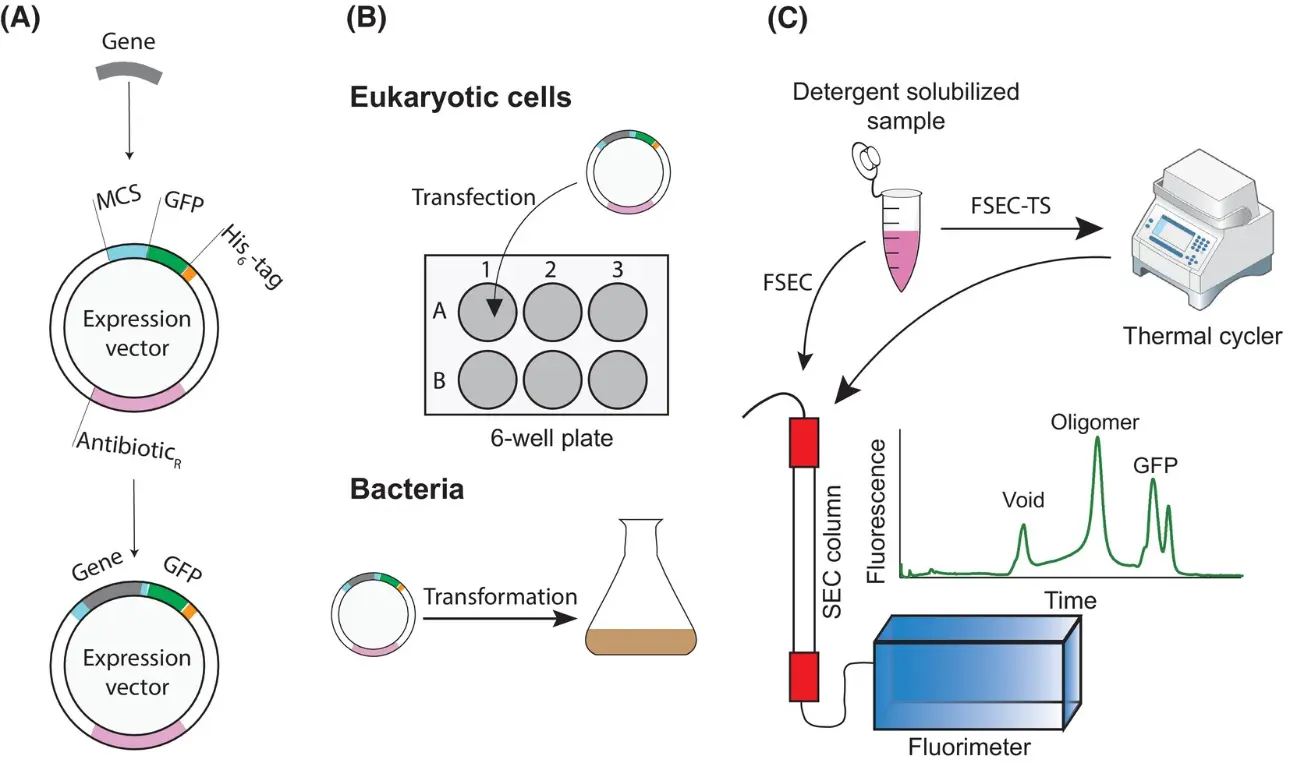
How Are GFP Fusion Proteins Created?
To create a GFP fusion protein, scientists use molecular cloning techniques to insert the GFP gene in-frame with the gene of the target protein. The resulting construct is introduced into cells using vectors such as plasmids or viral systems.
The fusion can be placed:
- At the N-terminal (beginning) of the target protein
- At the C-terminal (end)
- Or sometimes within internal loops, depending on protein structure and function
Once expressed, the fusion protein emits green fluorescence, allowing direct observation through fluorescence microscopy.
Why Use GFP Fusion Proteins?
1. Protein Localization
Determine where a protein is located within the cell (nucleus, cytoplasm, membrane, organelles, etc.).
2. Protein Dynamics
Track how a protein moves during processes like mitosis, migration, or signaling events.
3. Protein Expression
Visualize when and how much of a protein is produced under different conditions or treatments.
4. Real-Time Imaging
Study proteins in living cells or organisms in real time without the need for destructive staining.
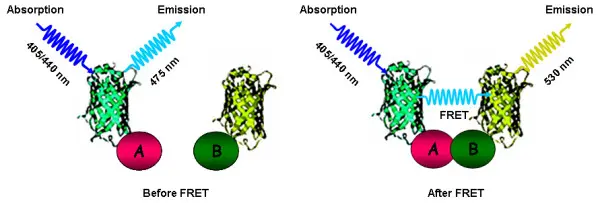
5. Protein-Protein Interaction (with FRET)
GFP fusion can be combined with other fluorescent proteins (e.g., CFP/YFP) for FRET-based analysis of interactions between proteins.
Live Cell Imaging with GFP: Techniques and Protocols
Live-cell imaging using Green Fluorescent Protein (GFP) is a cutting-edge technique that allows scientists to visualize and track dynamic biological processes in real time within living cells. Thanks to GFP’s natural fluorescence and compatibility with living systems, it has become a gold standard in cellular imaging.
What Is Live-Cell Imaging?
Live-cell imaging refers to the process of using time-lapse microscopy to monitor the behavior, localization, and interactions of proteins or organelles in living cells over time—without fixing or staining the sample.
GFP is ideal for this, as it:
- Requires no external substrates or cofactors
- Is non-toxic and non-invasive
- Provides real-time visualization of gene and protein activity
1. Widefield Fluorescence Microscopy
Basic technique using mercury or LED light sources to excite GFP. Best for general observations but with limited depth and resolution.
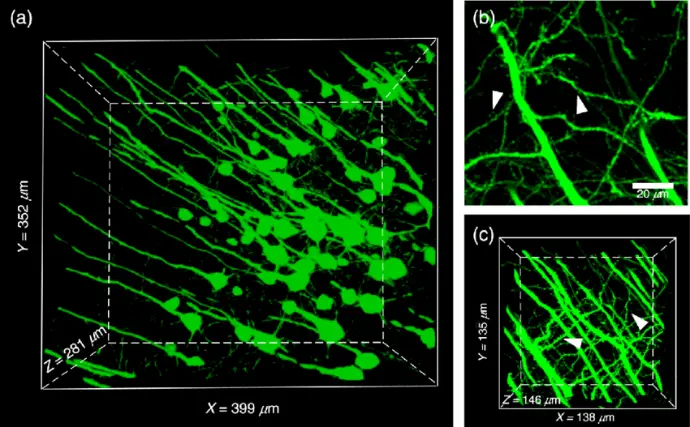
2. Confocal Laser Scanning Microscopy
Uses a laser to scan a focused point across the sample. Provides high-resolution and depth-selective imaging—ideal for subcellular localization studies.
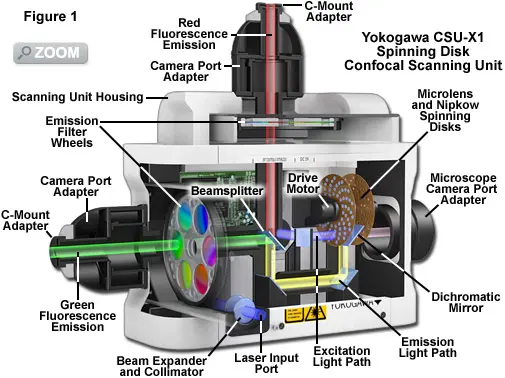
3. Spinning Disk Confocal Microscopy
Faster and gentler than laser scanning, suitable for real-time imaging of rapid cellular processes.
4. Two-Photon Microscopy
Used for deep tissue imaging with reduced phototoxicity. Ideal for imaging cells in whole organisms (e.g., zebrafish, mice).
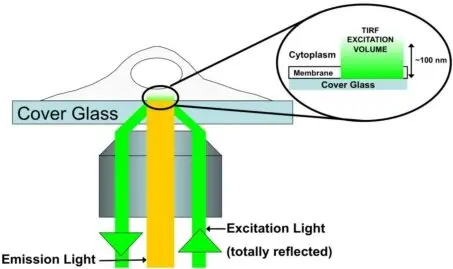
5. TIRF Microscopy (Total Internal Reflection Fluorescence)
Specialized for studying membrane dynamics and events at the cell surface with extremely high resolution.
Green Fluorescent Protein (GFP) has revolutionized molecular and cellular biology. However, other fluorescent proteins like mCherry, RFP, YFP, and CFP are widely used for multiplex imaging and specific applications.
🧪 GFP vs. mCherry
Feature
|
| |
Emission color | Green (
~509 nm)
| Red ( ~610 nm) |
Excitation wavelength | 488 nm | 587 nm |
Photostability | Moderate | High |
Brightness | High | Medium |
Maturation speed | Fast | Medium |
Cytotoxicity | Low | Very low |
GFP is ideal for general use and live-cell imaging, while mCherry is preferred when red fluorescence is needed, or when using dual-labeling techniques to reduce spectral overlap.
🔴 RFP (Red Fluorescent Protein)
RFP and its derivatives (like DsRed, TagRFP) offer intense red fluorescence, useful in multi-color imaging. However, early versions suffered from aggregation and slow maturation, which have been improved in engineered versions.
🔵 CFP and YFP
- CFP (Cyan Fluorescent Protein) is used in FRET (Förster Resonance Energy Transfer) experiments as a donor.
- YFP (Yellow Fluorescent Protein) is the typical FRET acceptor and is useful in protein interaction studies.
🧬 Choosing the Right Fluorescent Protein
When selecting a fluorescent protein, consider:
- Spectral properties (excitation/emission overlap)
- Photostability (for long-term imaging)
- Brightness
- pH stability
- Maturation time
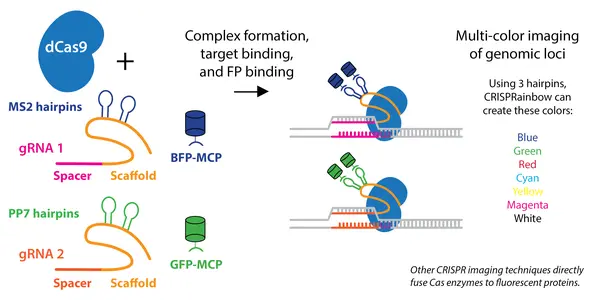
GFP in CRISPR/Cas9 Gene Editing
Marker Applications
Tracking gene integration: Cells that glow green indicate successful GFP knock-in.
Sorting modified cells: GFP-positive cells can be isolated via FACS (Fluorescence-Activated Cell Sorting).
Promoter activity: GFP linked to a promoter reveals its activity in real time.
Gene silencing tests: CRISPR-induced mutations can turn off GFP, indicating gene knock-out.
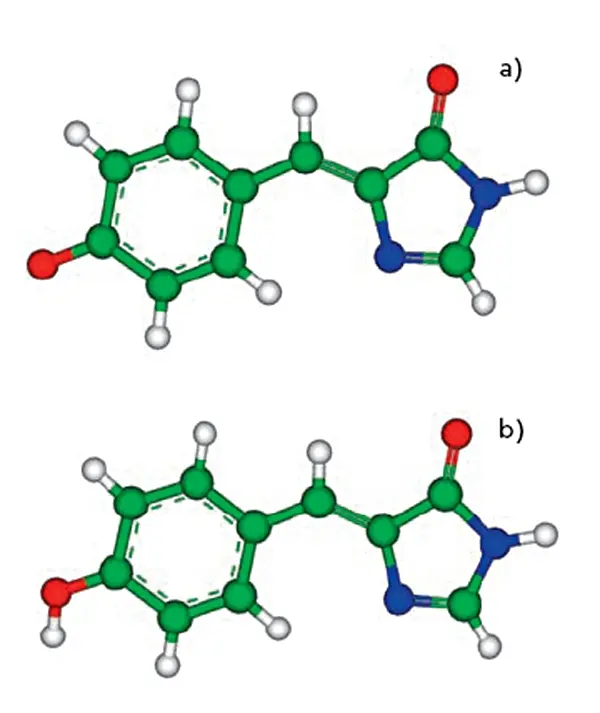
Spectral Properties of GFP Emission/Excitation Profiles
Understanding the spectral characteristics of GFP is crucial for proper microscope setup and avoiding spectral overlap in multi-label experiments.
Key GFP Properties:
Excitation peak: ~488 nm
Emission peak: ~509 nm
Quantum yield: ~0.60 (very bright)
Photostability: Moderate
Variants Spectral Differences:
CFP: Excites at 433 nm, emits at 475 nm
YFP: Excites at 514 nm, emits at 527 nm
mCherry: Excites at 587 nm, emits at 610 nm
📌 GFP’s spectral separation from red and blue fluorophores allows for multicolor live-cell imaging without cross-talk.
Photobleaching and Photostability in Fluorescence Imaging
Photobleaching is the irreversible loss of fluorescence due to prolonged light exposure. It can compromise long-term imaging.
Why Photobleaching Matters:
- Affects time-lapse and live-cell studies
- Can reduce signal-to-noise ratio
- Impacts quantitative fluorescence analysis
GFP Photostability:
- Moderate photostability under standard conditions.
- Enhanced by using antifade reagents, lower laser intensity, or newer variants like eGFP.
Best Practices to Reduce Bleaching:
- Minimize exposure time
- Use high-sensitivity cameras
- Apply real-time autofocus and automated imaging workflows
GFP-Tagged Vectors and Expression Systems
GFP-tagged vectors are plasmids designed to express proteins fused with GFP. This allows real-time visualization of protein expression, localization, and trafficking.
🔹
- Fusion proteins for subcellular localization
- Inducible expression systems
- Stable cell line creation
- Tracking promoter activity
🔹
- pEGFP, pGFP-N1/C1
- Lentiviral GFP vectors (for stable expression)
- AAV GFP vectors (used in gene therapy research)
🔹
- Lipofection
- Electroporation
- Viral transduction
These systems are essential for live-cell tracking, drug testing, and cell lineage tracing in biomedical research.